Abstract
Background: Patients (pts) with multiple myeloma (MM) who do not achieve complete response (CR) with primary therapy, including autologous hematopoietic cell transplantation (AHCT), have worse long term outcomes. Preclinical and clinical evidence suggests that the immune checkpoint programmed death-1 (PD-1) receptor/PD-1 ligand axis plays an important role in suppressing immune surveillance against MM, especially after AHCT. Thus, we hypothesized that the administration of pembrolizumab, an anti-PD-1 antibody, to disrupt the PD-1/PD-L1 axis to augment T and NK cell function during the lymphodepleted state post-AHCT (lack of competitors: cytokine sink) when PD-1 expression on effector cells peaks and MM tumor burden nadirs, may result in therapeutic efficacy in MM.
Patients and Methods: We conducted a phase 2, two-site, single-arm study of post-AHCT pembrolizumab in MM pts who had not achieved pre-AHCT CR to induction therapy. Pembrolizumab was administered 200 mg IV fixed dose every 3 weeks, starting day+14 after AHCT (upon engraftment) for 9 doses (day+180). The primary endpoint was CR rate after last treatment in pts receiving ≥2 doses (evaluable pts). Bone marrow (BM) minimal residual disease (MRD) testing was checked using flow cytometry in each institution (sensitivity 1 in 30,000 - 1 in 200,000). Pts aged 18-70 years with MM within 12 months after diagnosis, of any molecular risk group and with <CR status after induction therapy were eligible and received high-dose melphalan (140-200 mg/m2 IV), followed by AHCT and post-transplant maintenance therapy with lenalidomide 5-15 mg/day per standard of care.
Results: Thirty-two pts were enrolled but 3 withdrew consent prior to receiving first dose. For the 29 pts who received pembrolizumab, there were 19 males and 10 females and the median age was 59 years (range, 49-70). Four pts (14%) had light chain MM. ISS stages were stage I and II in 10 pts each (34%) and stage III in 9 pts (33%). All pts except 1 received a triple-regimen primary therapy, with a median of 4 cycles (range, 2-7). Twenty pts (69%) achieved partial response (PR) and 9 (31%) very good PR pre-AHCT. The median post-transplant day of initiation of pembrolizumab was 14 days (range, 13-19). The median number of doses received was 9 (range, 1-9). Among these 29 pts, grade 3 toxicities leading to discontinuation included infusion reaction and colitis in 1 pt each and grade 2 radiculopathy in another pt. Overall, another 12 immune-mediated adverse reactions that did not lead to discontinuation were grade 1-2 events including 4 infusion reactions, 2 colitis, 2 skin rashes, 2 hypothyroidism and grade 3 events of acute kidney injury and hepatitis (1 pt each). There were no primary or secondary graft failures, and the median time to neutrophil and platelet engraftment days were 11 days (range, 6-13) and 12 days (range, 8-39), respectively.
Twenty-six pts were considered evaluable for response assessment. Twenty-four pts started lenalidomide maintenance at the median of 103 (range, 60-154) days post-AHCT. For the other 2 pts, one had progressive disease at day 100 and the other withdrew consent.
Of the 23 pts who had a complete end-of-treatment (EOT) response evaluation by serology alone (non-CR) or both serology and bone marrow (BM) study (morphology and flow cytometry) (CR); the CR rate was 31% (7/23). In the remaining 3 pts, 2 pts with serologic CR did not have a confirmed BM at EOT study while 1 pt had no EOT evaluation. The MRD-negative rates by BM flow cytometry were 44% (11/25) at day100 and 67% (12/18) at EOT. With the median follow-up of 11.3 months (range, 3-24), none of 7 pts achieving CRs has relapsed; 3 progressed by EOT and one did by 8 months after initial EOT response. The estimated 1-year overall survival and progression-free survival are 100% and 87% (95% CI; 74%-100%), respectively.
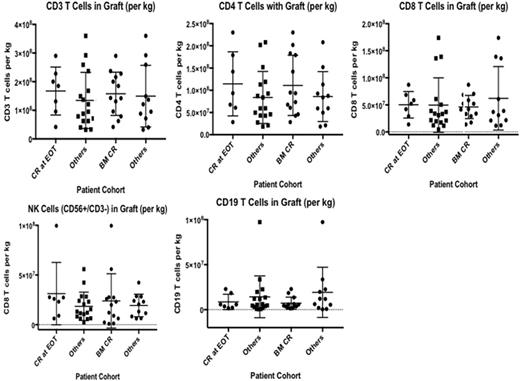
There was no correlation between the numbers of T cells, NK cells or B cells in the grafts infused back and clinical response (CR and BM MRD-negative disease) (Figure).
Conclusions: The addition of pembrolizumab in the early post-AHCT period was safe, as was the combined administration with standard-of-care low-dose lenalidomide maintenance. Pembrolizumab resulted in CR rate of 31% at 6 months, including a very encouraging 67% rate of BM MRD-negative state, in these patients not in CR pre-AHCT. Concurrent administration of pembrolizumab and low-dose lenalidomide maintenance is able to deepen the responses observed after AHCT.
Pawarode: AMGEN: Research Funding; MERCK: Research Funding. D'Souza: Merck: Research Funding; Prothena: Research Funding; Celgene: Consultancy. Dhakal: Celgene: Honoraria. Hamadani: Sanofi Genzyme: Research Funding, Speakers Bureau; Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding; Celgene, Cellerant, Jansen, MedImmune: Consultancy. Shah: Oncosec: Equity Ownership; Exelixis: Equity Ownership; Geron: Equity Ownership; Jazz Pharmaceuticals: Consultancy. Hari: Janssen: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Honoraria, Research Funding; Novartis: Consultancy; Spectrum: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal